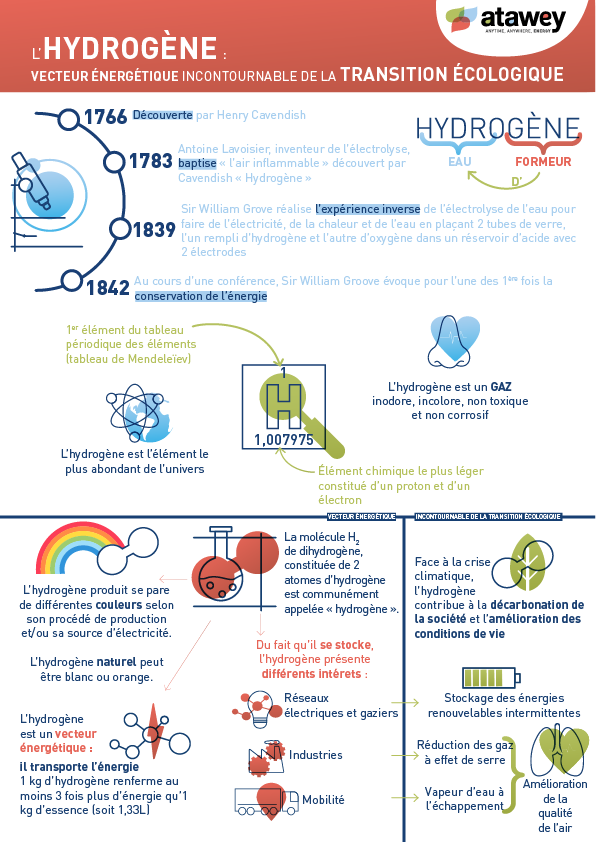
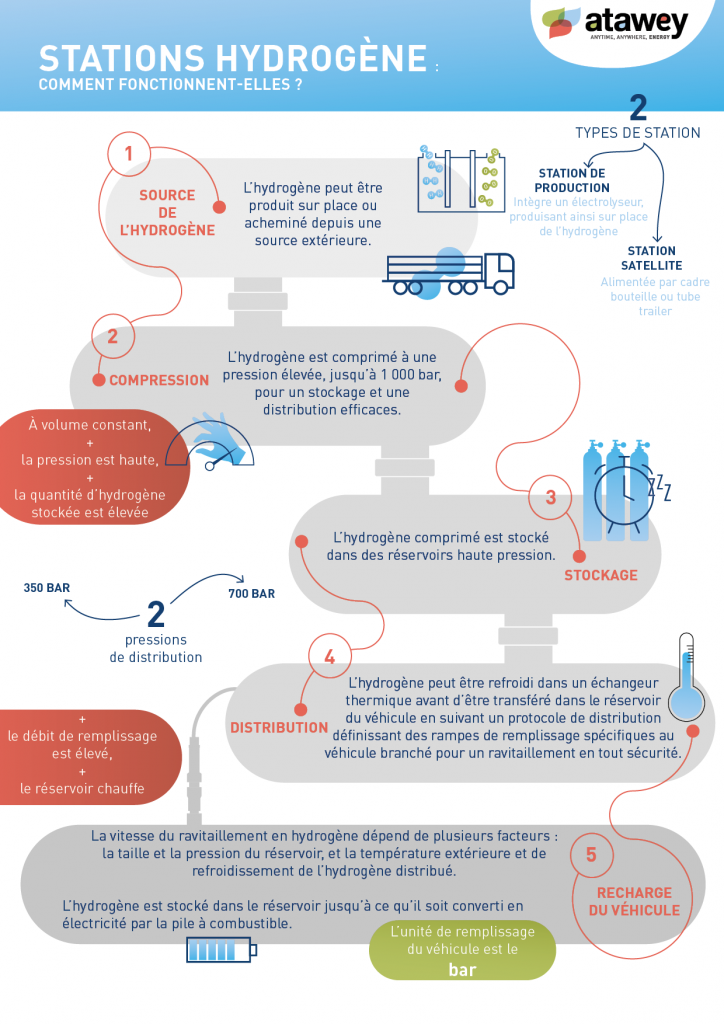
Natural Hydrogen: A Promising Revolution for the Energy Transition
In a world seeking clean and sustainable energy alternatives, natural hydrogen, also known as white hydrogen, is emerging as a revolutionary solution. Unlike green, blue, or gray hydrogen, its production requires neither renewable electricity nor intensive chemical processing. This energy carrier, naturally generated through geological processes, holds significant potential to decarbonize strategic sectors while reducing costs and environmental impacts. This article delves into the promises and challenges of white hydrogen, highlighting initiatives in France, Europe, and globally, as well as its role in the energy transition.
An untapped natural potential
Natural hydrogen, or white hydrogen, is emerging as an innovative and strategic resource in the global energy transition. Formed through geological processes such as the oxidation of ferrous minerals or water radiolysis, it is continuously produced by the Earth over periods ranging from a few thousand to several million years, depending on specific geological conditions. Unlike other types of hydrogen, its production neither depends on intermittent electricity sources nor requires complex CO2 capture technologies, significantly reducing associated costs. Additionally, white hydrogen extraction boasts several environmental advantages:
- No fossil fuel dependency: Unlike gray or blue hydrogen, it does not rely on natural gas reforming, eliminating a major source of emissions.
- Continuous natural production: The Earth’s natural hydrogen generation processes provide a low-energy alternative to high-input methods like electrolysis.
- Minimal land and water use: Unlike green hydrogen, which requires significant water and land for renewable energy installations, white hydrogen extraction involves a smaller operational footprint.
Recent projections estimate that white hydrogen production costs could range between €1 and €1.5/kg in the coming years, well below the €2 to €9/kg expected for hydrogen produced by electrolysis by 2030. By comparison, gray hydrogen from fossil fuels currently costs between €1.5 and €3/kg but has a significantly higher environmental impact. These figures highlight the competitive advantage white hydrogen could represent for the energy and industrial sectors.
Natural Hydrogen: Promising initiatives in France and globally
In France, five exploration permits have been granted, targeting regions such as the Pyrenees, the Albigeois Plain, and French Guiana. These areas have been identified for their favorable geological potential, particularly due to the presence of rock formations rich in iron and magnesium, which facilitate natural hydrogen generation. Preliminary estimates suggest that these areas could supply up to 20% of France’s national hydrogen demand by 2050, depending on technological developments and exploration progress.
In the Pyrenees, projects are exploring magmatic rocks and active serpentinization zones, while in French Guiana, studies focus on Precambrian formations rich in iron. In the Albigeois Plain, promising geochemical indicators reveal significant amounts of dissolved hydrogen in deep aquifers. These deposits could be exploited to power local industries, reduce fossil fuel imports, and support the decarbonization of heavy transport. Initial results from exploratory drilling, expected by 2025, should confirm the commercial potential of these projects.
Across Europe, several emblematic projects are advancing the understanding of natural hydrogen:
- Kosovo: The “Banja Vuca” project in the Dinarides region covers 57 km², with feasibility results expected in 2024.
- Finland: Exploration in the Outokumpu Belt, supported by Bluejay Mining, is targeting formations known for their hydrogen generation potential.
- Poland: Preliminary studies in Lower Silesia focus on serpentinization in magnesium-rich formations.
- Ukraine: In the Donetsk Basin, researchers are investigating hydrogen generation under specific geothermal conditions.
- United Kingdom: The Scottish Highlands host studies of ultramafic zones where rock-water interactions produce hydrogen.
Globally, key initiatives further underscore the growing interest in white hydrogen. In Mali, the pilot project at Bourakébougou has already proven the existence of exploitable deposits, generating hydrogen directly usable for local energy production. In the United States, exploratory drilling has begun in Nebraska and Kansas, targeting areas such as the Nemaha Ridge, where preliminary geological studies indicate promising potential. In Australia, the Yorke Peninsula hosts pilot projects investigating serpentine-rich formations conducive to natural hydrogen generation .
According to a 2020 study by Zgonnik, over 465 geological occurrences of hydrogen have been identified worldwide, demonstrating a largely untapped global potential. These deposits include areas in Latin America (such as Colombia), Eastern Europe (notably Poland and Ukraine), and Africa (for example, Namibia). While many of these projects are still in exploratory stages, they could pave the way for commercial exploitation by 2030, supported by technological advances and investments estimated at several billion euros.
A ckey lever for the energy future
Despite its promise, white hydrogen faces several challenges, including the development of dedicated infrastructure for extraction, transportation, and storage, as well as the establishment of harmonized regulatory frameworks at the international level. Geological hydrogen storage offers a natural complement to its exploration and production, leveraging underground formations such as salt caverns and aquifers to store surplus hydrogen efficiently. This approach aligns with the development of hydrogen as an energy vector, enabling flexible supply for industrial needs and buffering seasonal energy demands. Projects like the HyGéo Project in Germany are already repurposing salt caverns for hydrogen storage, showcasing the feasibility of large-scale geological systems. In France, the potential for coupling natural hydrogen exploration with geological storage could create a resilient hydrogen ecosystem, reducing reliance on fossil fuels and stabilizing energy markets.
Preliminary estimates suggest that ongoing projects in France and across Europe could significantly contribute to climate objectives by providing a competitive and sustainable alternative to fossil fuels. If public and private initiatives continue at this pace, natural hydrogen and its associated storage infrastructure could become a major pillar of the global energy transition, particularly in industrial sectors and heavy mobility.