H₂: A Bit of Physics and Chemistry
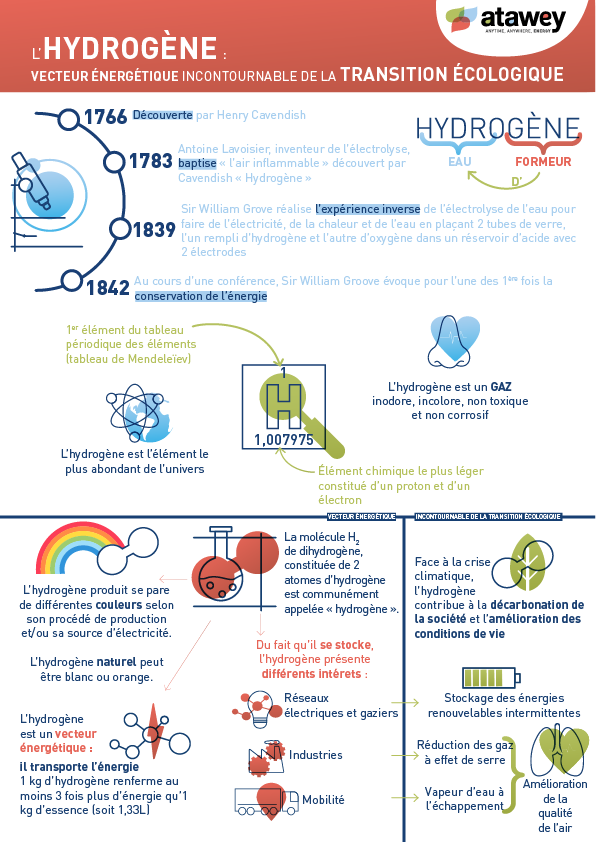
Hydrogen—the simplest and most abundant element in the universe—has a rich history that dates back to its discovery by British scientist Henry Cavendish in the 18th century. Since then, this molecule has undergone a remarkable evolution, transforming from a scientific curiosity into a key component of many modern applications, now developed within thriving H₂ ecosystems.
The Age of Enlightenment: The Discovery of Hydrogen
In 1766, British physicist and chemist Henry Cavendish made a major scientific breakthrough by discovering a unique gaseous substance during his experiments on acidity. Three years later, he published his findings, revealing the distinctive nature of this light gas, which he referred to at the time as “inflammable air.”
In 1783, French chemist Antoine Lavoisier recognised that Cavendish’s “inflammable air” was, in fact, a distinct chemical element that, when burned in the presence of oxygen, played a role in the formation of water. He therefore proposed the name “hydrogen” for this element, derived from the Greek words hydro (meaning “water”) and genes (meaning “creator” or “former”).
H₂: The Chemical Formula for Hydrogen
Why Is Hydrogen Referred to as H₂?
The molecular form of hydrogen is dihydrogen. As the name suggests, this molecule consists of two hydrogen atoms, which is why its chemical formula is written as H₂.
The Era of Chemical Renaissance: H₂ Gas Becomes an Energy Carrier
In 1839, Sir William Grove—a British lawyer and amateur chemist—developed the Grove cell, also known as the gas voltaic battery, by combining platinum electrodes with hydrogen and oxygen. This invention directly converted chemical energy into electricity without combustion, offering a clean and efficient alternative to traditional electricity generation methods.
In the following years, he published a series of papers and, during a lecture in 1842, he discussed the concept of energy conservation—making one of the earliest references to it, five years before Hermann von Helmholtz.
His gas cell would remain dormant for decades, but it would later emerge as a crucial precursor to the invention of the modern fuel cell.
Thus, the era of chemical renaissance was not only a witness to fundamental scientific discoveries, but also a catalyst for a new age in the field of energy—marked by the innovative use of hydrogen as an energy carrier.
Industrial Revolution: Expanding Uses of Hydrogen
In the 20th century, hydrogen became a vital resource in industry, with a wide range of applications that helped shape global manufacturing processes and production systems. One of its most significant uses is in ammonia production. This chemical reaction, known as the Haber–Bosch process, combines hydrogen with atmospheric nitrogen to produce ammonia—a key raw material in the manufacturing of fertilisers.
The oil industry has also incorporated hydrogen gas into its processes. In petroleum refining, for example, hydrogen is used to remove impurities and enhance the quality of petroleum products. One key application is the hydrodesulfurization process, which uses hydrogen to reduce the sulphur content in fuels—contributing to cleaner combustion and improved environmental performance.
Hydrogen: An Essential Gas to Meet the Needs of Modern Society
Hydrogen is also used in metallurgy to reduce metal ores such as iron, thereby contributing to steel production. This specific application, known as direct reduction, enables the extraction of metals from their oxides and plays a key role in the manufacturing of materials essential to numerous industries.
In the electronics sector, hydrogen plays a role in the production of semiconductors and advanced electronic components. Its use in these applications supports the ongoing advancement of information and communication technologies.
Hydrogen’s central role in fertiliser production, oil refining, and other industrial processes makes it an indispensable resource for meeting the growing needs of modern society.
Post-Industrial Era: H₂ in the Ecological Transition
While still widely used to decarbonise industry, hydrogen has emerged—at the turn of the 21st century—as a promising solution to environmental and energy-related challenges, making it a key player in the ecological transition. One of its main advantages lies in the decoupling of production and consumption: hydrogen can be produced during off-peak hours, stored safely, and used whenever needed.
This is why H₂ is now being used as a means of energy storage: it serves as an energy carrier to store renewable electricity generated from intermittent sources such as wind and solar. In doing so, it helps smooth out fluctuations in energy production, contributing to a more stable and reliable power supply to the grid.
Hydrogen (H₂): A Decarbonisation Solution for the Transport Sector
Hydrogen is also part of the solutions for decarbonising transport, offering zero CO₂-emission mobility and thereby helping to reduce the sector’s carbon footprint while also improving air quality.
For example, hydrogen can be used to power fuel cells that generate electricity for electric vehicles. In addition, hydrogen’s storage capacity is leveraged in these vehicles: the energy not immediately used by the electric motor is stored in a battery, which can then support the fuel cell when needed.
Hydrogen vehicles require dedicated infrastructure for refuelling: hydrogen stations. These rapidly developing stations are equipped with dispensers capable of injecting hydrogen gas under pressure directly into the vehicle’s tank.
Hydrogen is therefore emerging as a versatile player in the energy transition, offering innovative solutions to address the climate and energy challenges of the 21st century.
Did You Know?
Although hydrogen is a colourless and odourless gas, it’s still given a whole spectrum of colours!
Indeed, the colours of hydrogen are designations that refer to how it is produced and, in some cases, the type of electricity used in the process. “Green” hydrogen, for example, is produced using renewable or low-carbon energy sources—such as wind, solar, or water electrolysis.
Hydrogen: A Key Energy Carrier in the Ecological Transition
From its discovery by Cavendish to its modern-day applications, the story of hydrogen is a saga of scientific breakthroughs and technological innovation. As we continue to explore new ways to harness this versatile molecule, it is clear that hydrogen will remain a key player in the pursuit of a sustainable energy future.